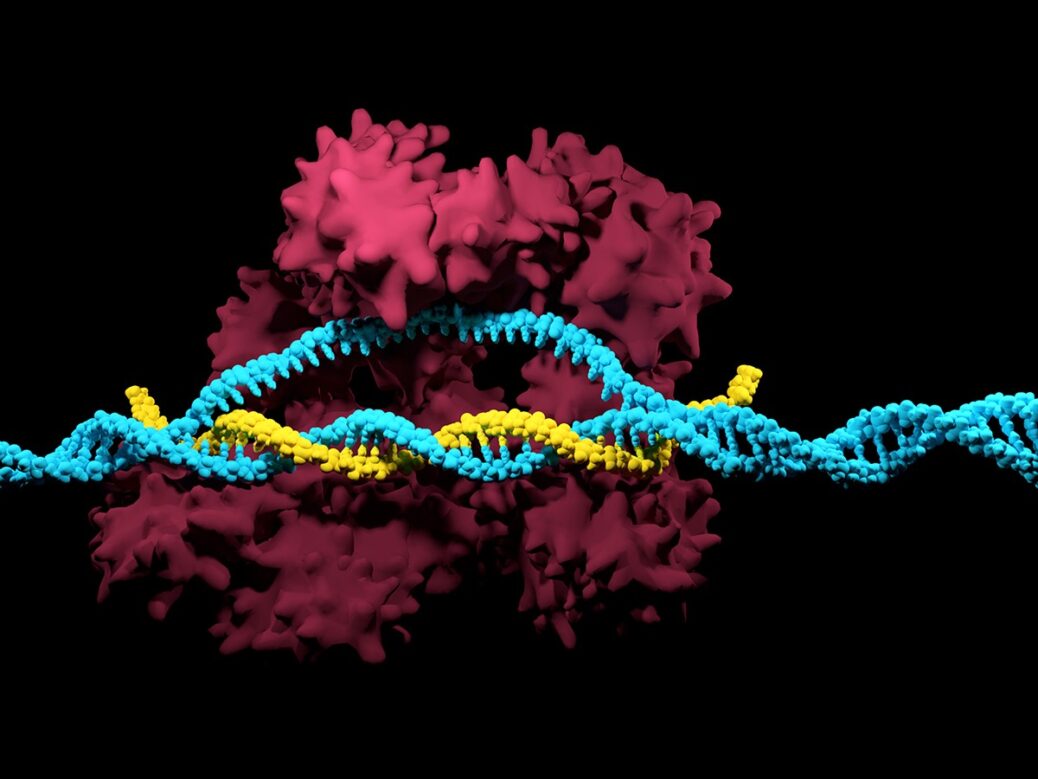
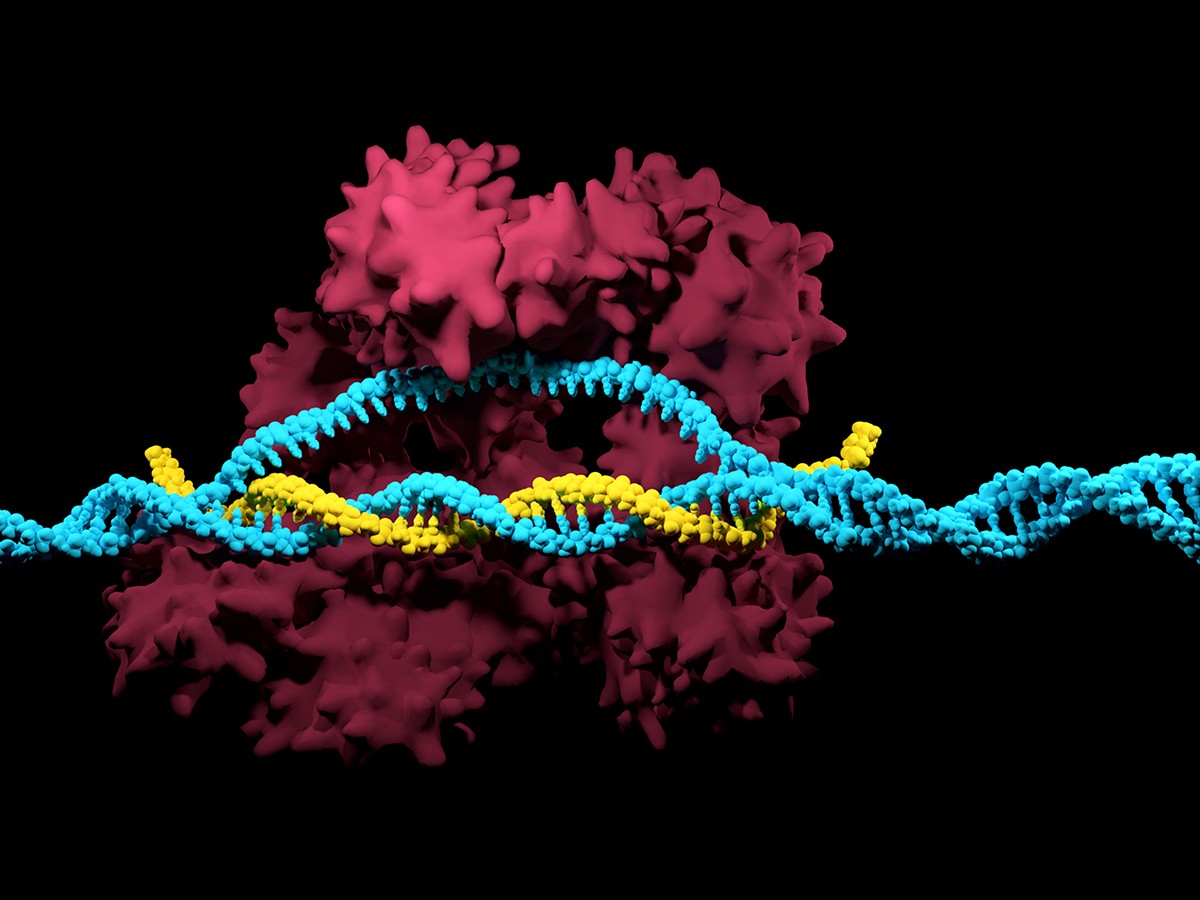
CRISPR, a gene-editing technology that allows scientists to “gene edit” DNA has been caught in a “who done it” legal pickle for the last four years as two “co-inventor” groups argue for patent rights. Intellectual Patent disputes are very intense as the winner is allowed to license or “commercialize” the patent for the next 20 years without competition (in theory as future disputes and infringements are common). The bottom line is that patent ownership allows for a significant competitive edge. Many times, research institutions – like pharmaceutical companies – are eager to for a ROI (Return On Investment) and then-some in order to sustain a competitive edge. Companies like Apple and Samsung are in constant legal standoffs, with each ruling leading to subsequent appeals and additional filings. The fees alone can capsize a company’s projected revenue streams making IP Litigation a very “bullish” type of practice that some lawmakers are attempting to regulate. While the more “organic” reason for IP protection is to protect the consumer from fraud all while championing innovation, the current status has in fact hampered R&D growth all the while mitigating an unfair advantage to bottom line or bust companies. The Human Genone Project remains a beacon of hope for what true collaboration can accomplish. The only problem – with this instance – we are embarking on an entirely new paradigm
CRISPR isn’t your average innovation.
We’re talking DNA.
Not Consumer Electronics or Agriculture.
DNA.
The projectile for future innovation is astronomical.
In this case, we have two research institutes claiming to be the original inventor.
Broad Institute and the Massachusetts Institute of Technology
According to Broad Institute website its mission is:
The institute was founded to seize the opportunity that arose from the Human Genome Project — the international effort that successfully deciphered the entire human genetic code. Despite that accomplishment, scientists knew they still lacked a clear understanding of the genetic basis of disease, and how to translate that understanding into more effective prevention, diagnosis, and treatment.
To reach these goals, it was clear that a new type of research institution had to be created. The traditional academic model of individual laboratories working within their specific disciplines was not designed to meet the emerging challenges of biomedicine. To gain a comprehensive view of the human genome and biological systems, they instead had to work in a highly integrated fashion.
That meant working in nimble teams that combined biology, chemistry, mathematics, computation, and engineering with medical science and clinical research. It also meant working at a scale usually seen in industry, with access to world-class infrastructure. At the same time, this institution had to foster an atmosphere of creativity, risk-taking, and open sharing of data and research. Finally, this new model needed to seek collaborations beyond its borders.
In short, the traditional research model present within academia did not serve the purpose or production output of biomedicine. A more collaborative approach was required within a field that usually is very protective of new ideas in order to be the first to publish in peer to peer publications (this being the basis for additional government and private funding).
UC Berkely College of Chemistry and Biomolecular Engineering
UC Berkely College of Chemistry and Biomolecular Engineering share similar goals as BROAD Institute:
The chemical and biomolecular engineering faculty members have established world-renowned research programs in fields such as thermodynamics, surface catalysis, electrochemical processes, fluid mechanics, separation and transport processes, polymer processing, and control systems, and in such promising research fields as biochemical engineering, nanotechnology, and the study of electronic and optical materials.
Faculty members in the chemistry department are involved in research programs that encompass all areas of chemical research, including both the traditional fields of analytical, inorganic, organic, and physical chemistry, as well as such diverse areas as nanoscience; nuclear, biophysical, materials and atmospheric chemistry; and structural and chemical biology.
Broad Institute and the Massachusetts Institute of Technology 1 v. Berkeley 0.
On Monday, the US Court of Appeals for the Federal Circuit issued a decisive ruling on the rights to CRISPR-Cas9 gene editing—awarding crucial intellectual property spoils to scientists at the Broad Institute of Cambridge, Massachusetts. Although Broad Institute and the Massachusetts Institute of Technology might’ve won the battle, the war is far from over. According to an article published on 09.11.208 by Wired Magazine, CRISPR’S EPIC PATENT FIGHT CHANGED THE COURSE OF BIOLOGY
In addition to the Broad Institute’s claims, UC-Berkeley also has to contend with another foundational patent for Crispr-Cas9 gene editing filed before anyone else in March 2012, by Virginijus Šikšnys, a Lithuanian scientist who shares the prestigious Kavli Prize with Berkeley’s Jennifer Doudna and The University of Vienna’s Emmanuelle Charpentier for their early work on Crispr. The USPTO has since granted his patent. UC didn’t know about it at the time of its own filing because of an 18-month secrecy statute surrounding new applications. “If this was a choose-your-own-adventure book, they just turned all the wrong pages,” says Sherkow.
– Meghan Moltini
First, let’s take a look at a brief timeline of what went down in CRISPR.ville
UC Berkeley v. The Broad Institute and the Massachusetts Institute of Technology, Legal Timeline
Source: Becker’s Health IP and CIO Report // UC Berkeley and Broad Institute’s legal dispute over CRISPR ownership: A timeline of events // June 21, 2018
May 2012: UC Berkeley filed a patent application with the USPTO for the use of CRISPR-Cas9 to edit genes in various types of cells. The application was based on a landmark research study by a team of UC Berkeley researchers, which was slated to be published in June.
June 2012: The research team from UC Berkeley published what many biotechnology experts cite as the first academic paper on CRISPR-Cas9. The study, published in the journal Science, detailed how CRISPR-Cas9 may be exploited to edit genes. The research team was led by UC Berkeley biochemist Jennifer Doudna, PhD, who some experts credit with creating CRISPR, according to Futurism.
December 2012: The Broad Institute and the Massachusetts Institute of Technology, also based in Cambridge, Mass., filed a patent application for the use of CRISPR-Cas9 to modify DNA in eukaryotic cells. The patent is based on research conducted by Feng Zhang, PhD, a molecular biologist affiliated with both the Broad Institute and MIT.
April 2014: The USPTO granted the patent filed in December 2012 to the Broad Institute, MIT and Dr. Zhang. The patent, titled “CRISPR-Cas systems and methods for altering expression of gene products,” covers a method of editing plant and animal DNA using CRISPR-Cas9.
-
However, UC Berkeley contested the USPTO’s decision to grant the Broad Institute the patent, arguing the institute’s application was too similar to the patent application UC Berkeley had applied for in May 2012. As a result, UC Berkeley asked the USPTO to deem the Broad Institute’s patent invalid, according to STAT. The university also asserted Dr. Doudna’s research team was the first group to invent the CRISPR-Cas9 gene-editing technology.
-
The Broad Institute has maintained the patent it received draws on Dr. Zhang’s original research using CRISPR-Cas9 to edit plant, animal and human cells, which — while published in Science in February 2013 — was submitted in October 2012 and serves as a culmination of his work that began in early 2011. The Broad Institute has also argued Dr. Zheng’s study marked the first use of CRISPR-Cas9 for mammalian genome editing.
February 2017: The USPTO ruled in favor of the Broad Institute, upholding the institute’s patent on editing DNA in plants and animals. In its decision, the USPTO said Dr. Zhang’s discovery was not an “obvious” extension of Dr. Doudna’s research as UC Berkeley had argued, according to Gizmodo. Dr. Doudna’s team appealed the decision.
April 2018: The U.S. Court of Appeals for the Federal Circuit heard oral arguments from UC Berkeley, during which the university attempted to prove the USPTO had not had “substantial evidence” to support its February 2017 finding, STAT reports. The court is expected to release a ruling on the case this summer.
June 2018: The USPTO granted a team of UC Berkeley researchers, including Dr. Doudna, the university’s first-ever patents related to CRISPR gene editing — one patent that covers the use of CRISPR-Cas9 to edit single-stranded RNA, and a second patent that covers the use of CRISPR-Cas9 for editing genome regions of 10 to 15 nucleotides long, according to STAT.
Let’s take a step back:
What is CRISPR?
CRISPR technology is a simple yet powerful tool for editing genomes. It allows researchers to easily alter DNA sequences and modify gene function. Its many potential applications include correcting genetic defects, treating and preventing the spread of diseases, and improving crops. However, its promise also raises ethical concerns.
Need a little more? Check out the 2-minute video below:
source: QUESTIONS AND ANSWERS ABOUT CRISPR by Feng Zhang
Sounds simple so far. But what are the implications? Give me one real world scenario that involves CRISPR and its potential.
Sure. How about eight. According to Digital Trends, article 8 Amazing CRISPR projects that could change life as we know it
1. MALARIA-RESISTANT MOSQUITOS
To help battle this, researchers from Johns Hopkins University have used CRISPR-Cas9 gene editing to engineer mosquitos that are resistant to the malaria parasite. By deleting a gene that enables malaria to survive in the mosquito’s gut, the parasite is left unable to survive for long enough to be a danger to humans.
2. A LIMITLESS SUPPLY OF TRANSPLANT ORGANS
There’s an enormous, deadly shortage of transplant organs worldwide. Could CRISPR gene editing help? Quite possibly yes, according to one international research initiative with the goal of using gene editing on pigs to turn them into safe organ donor candidates for humans.
3. ENCODING GIFS IN YOUR DNA
Researchers at Harvard University recently showed that it is possible to use CRISPR to accurately encode images and even movies into DNA. The footage in question is a 36 x 26-pixel GIF showing a galloping horse filmed by motion picture pioneer Eadweard Muybridge all the way back in 1878.
4. SAVING THE WORLD’S CORAL REEFS
Looking to change that, an international research project is using CRISPR to examine exactly how and why environmental changes hurt coral reefs. No, there are no immediate plans to create a CRISPR-enabled breed of super coral, but understanding coral genes will help researchers to get to grips with phenomena like coral bleaching. And hopefully start making efforts to reverse it.
5. MORE EFFICIENT CROPS
The world’s population is rapidly increasing, and that brings a range of challenges when it comes to how best to feed everyone. Research aided by the Bill & Melinda Gates Foundation has demonstrated that it is possible to use CRISPR to improve the efficiency of how crops use water by 25 percent — without compromising their yield in the process.
6. HEAT-RESISTANT COWS
By isolating the specific DNA segments which allow it to regulate its body temperature so effectively, they hope to make other types of heat-resistant bovine. All without sacrificing taste, of course! Researchers from the University of Florida Institute of Food and Agricultural Sciences are trying to use genetic engineering to create heat-resistant cows which can thrive in warmer environments.
7. CURING ALS or Lou Gehrig’s
Researchers at the University of California, Berkeley have shown that it is possible to disable the defective gene which triggers ALS in mice. Although they were not able to get rid of the disease permanently, their treatment extended the mice’s life span by 25 percent.
8. HOME DISEASE DIAGNOSIS
“Similar to a search engine, our scientists enter a code into the guide RNA to find the matching nucleic acid (DNA or RNA) strand in the disease,” Mammoth CEO Trevor Martin told Digital Trends. “Once the code is found, instead of only snipping the strand of matched RNA or DNA like one would for editing, it also has a collateral effect on reporter molecules that release a color to visually show the presence of the disease.”
source:
Digital Trends // 8 Amazing CRISPR projects that could change life as we know it // by: Luke Dormehl / @lukedormehl
MyIpLife will keep you up to day on the legal and ethical uprisings as they occur. We have a vested interest in CRISPR and its unfurling and look forward to witnessing its full potential commercially within the healthcare industry and beyond. For future articles, information, and facts about CRISPR please follow our thread here: MYIPLIFE CRISPR ALERT
Latest posts by HuFi (see all)
- CRISPR Patents! And the Winner IS! - October 9, 2018
- Is it Possible to Patent Your Genome? - November 21, 2017
- “Mergence” How Will Human Fidelity Affect Posthumans? - February 8, 2016